Prostate Cancer Journal
" ... the medium through which I will monitor my treatment in the battle against cancer"
Wednesday, 27 November 2013
Short-Course ADT Passes Test in High-Risk Prostate Cancer
Sunday, 29 September 2013
The following are treatment options for castrate-resistant prostate cancer (also called hormone-refractory prostate cancer). The types of treatments given are based on the unique needs of the person with cancer.
If a man is only taking an LHRH agonist, an anti-androgen (usually bicalutamide) will be added when there are signs of hormone-refractory growth (usually a rising PSA). The LHRH is not stopped. The PSA will often come down for a period of weeks or months before rising again. When it rises, the anti-androgen is stopped but the LHRH is continued.
The most common chemotherapy drugs used to treat prostate cancer are:
- docetaxel (Taxotere)
- mitoxantrone (Novantrone)
- cabazitaxel (Jevtana)
The most common chemotherapy combinations used are:
- docetaxel and prednisone – This combination reduces pain, improves quality of life and increases survival.
- mitoxantrone (Novantrone) and prednisone (Deltasone) – This combination reduces pain and improves quality of life.
- cabazitaxel (Jevtana) and prednisone – This combination prolongs survival in castrate-resistant prostate cancer.
- short course of treatment (1–10 treatments) to relieve bone pain
- not given for 4–6 weeks after a transurethral resection of the prostate (TURP), to reduce the risk of scarring in the urethra (urethral stricture)
The type of bisphosphonate used with hormone-refractory prostate cancer is zoledronic acid (Zometa). It decreases bone-related complications in men with prostate cancer.
Transurethral resection of the prostate (TURP) may be offered for hormone-refractory prostate cancer. This type of surgery is used to relieve urinary symptoms caused by the prostate tumour (palliative surgery).
References
American Cancer Society. Prostate cancer overview. (2011, May 4). Detailed Guide: Prostate Cancer. Atlanta, GA: American Cancer Society. Retrieved from: http://www.cancer.org/.
de Bono,J.S., Oudard,S., Ozguroglu, M., et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. (2010, October 2). Lancet. New York, NY: Elsevier, Inc. Retrieved from: http://www.thelancet.com/journals/lancet/issue/current.
Fizazi K, Carducci M, Smith M,et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. (2011, March 5). Lancet. New York, NY: Elsevier, Inc. Retrieved from: http://www.thelancet.com/journals/lancet/issue/current.
Prostate Cancer Treatment (PDQ®). National Cancer Institute. (2010, August). National Cancer Institute (NCI). Bethesda, MD: National Cancer Institute. Retrieved from: http://www.cancer.gov.
Prostate cancer. National Comprehensive Cancer Network. (2010). NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network (NCCN).
Ross, P. L., Carroll, P. R., Small, E. J., et al. Prostate. Ko, A. H., Dollinger, M., & Rosenbaum, E. (2008). Everyone's Guide to Cancer Therapy: How Cancer is Diagnosed, Treated and Managed Day to Day. (5th Edition). Kansas City: Andrews McMeel Publishing. pp. 789-806.
Zelefsky MJ., Eastham JA, Sartor OA, et al. Cancers of the genitourinary system: cancer of the prostate. Devita, V. T., Jr., Lawrence, T. S., & Rosenberg, S. A. (2008). Cancer: Principles & Practice of Oncology. (8th Edition). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. 40.6: 1392-1451.
Source - Canadian Cancer Society:
http://www.cancer.ca/en/cancer-information/cancer-type/prostate/treatment/castrate-resistant-prostate-cancer/?region=on
Monday, 26 August 2013
Managing the Patient With a Rising PSA Following ADT
Unfortunately, the natural history of these patients is poorly understood. Numerous factors such as the lactate dehydrogenase, alkaline phosphatase, hemoglobin, and Eastern Cooperative Oncology Group or Karnofsky performance status have been used to predict prognosis,[39,40] while others have suggested that a detailed PSA history alone may be enough to stratify risk and predict prognosis.[41]
Because the velocity of PSA changes may be different before and after ADT, reflecting changes in tumor kinetics after treatment, intermediate end points based on the ratio of the post- to pre-ADT slope are being considered in clinical trials as an end point for comparing systemic therapy in patients with the "lethal phenotype" (ie, PSA doubling time < 3 months).
Nevertheless, until PSA doubling time and/or calculated pre- and post-ADT PSA velocities as predictors of survival in patients are widely accepted, a 50% decline in PSA is typically considered the benchmark for response to ADT, while PSA increases of a minimum of 5 ng/mL with two consecutive increases of 25% after ADT indicate clinical disease progression.[2,38,42]
Treatment Strategies
Castrate patients with a rising PSA present unique and complex challenges for the treating physician. Frequently, ADT continues to be employed while additional therapeutic strategies are explored. At this stage of disease, the establishment of a true multidisciplinary care team is essential, including the coordinated involvement of a urologist, an oncologist, and a radiologic imaging specialist.Before any additional therapeutic regimens can be considered, maintenance of castrate levels of testosterone (< 50 ng/mL) should be confirmed, as the presence of a rising PSA may in fact indicate that ADT is not sufficiently suppressing testosterone production and/or action and that additional androgen suppression therapy is warranted.[2]
Even if androgen independence is established despite castrate levels of testosterone and the rising PSA levels indicate biochemical failure, the tumor might respond to secondary hormonal manipulation.
In patients who had been treated with an antiandrogen, withdrawal of the agent while maintaining castrate levels of testosterone has been associated with PSA responses, as well as with symptomatic and objective responses, in about 25% of patients. The duration of response is typically 3-4 months, but some cases have lasted for several years.[2] This response has been noted with flutamide, bicalutamide, and nilutamide[43] and may result from mutations of androgen receptors.[44]
Thus, a trial of antiandrogen withdrawal may be reasonable for certain patients, particularly before initiating more toxic therapy. Although it is unclear whether the same antiandrogen would still be effective if reintroduced at a later point, cross-reactivity generally does not exist among the antiandrogens: both bicalutamide and nilutamide have shown some activity as secondary antiandrogens following resistance to flutamide.[45-47]
Adrenal androgen inhibitors, most commonly ketoconazole, also have a role as second-line hormonal agents. Approximately 10% of circulating androgens derive from the adrenal glands, and a higher proportion of adrenal androgens may be in prostate cancer cells.[2] Ketoconazole has been shown to decrease serum PSA by 50% with high doses[48] and low doses,[49] but the duration of response is short, with one study finding a median PSA response duration of only 3.5 months.
Similarly, glucocorticoids have also shown some effect in patients with AIPC. In several trials comparing hydrocortisone alone with hydrocortisone and a study treatment, disease response was seen in the hydrocortisone-alone arms.[50-53] However, as with ketoconazole, the duration of response tends to be rather short and further progression of disease is rapidly seen.
A more promising approach has been seen with chemotherapeutic agents. Chemotherapy with cytotoxic agents historically has had a minor role in the treatment of prostate cancer. In 1985, Eisenberger and colleagues reviewed 17 randomized clinical trials and found complete and partial response rates in only 4.5% of patients.[54] In 1993, in another large review, the overall response rate with chemotherapy was 8.7%.[55]
Switching tactics, researchers began to measure quality-of-life parameters in this population, and the use of mitoxantrone showed significant palliative benefits,[51] which led to its approval by FDA for use in patients with AIPC. Unfortunately, aside from its palliative benefits, it has shown only modest activity in measurable disease and lacks the ability to confer a significant survival advantage in patients with AIPC.[52]
By contrast, two phase 3 trials with docetaxel, one pairing with prednisone and the other pairing with estramustine, showed significant improvement in both survival and palliation in patients with AIPC when compared with the standard mitoxantrone/prednisone regimen.[56,57] These trials led to the recent approval by FDA of docetaxel in combination with prednisone every 3 weeks in patients with AIPC.
Newer agents targeting apoptosis, growth factors, angiogenesis, tumor-associated antigens, and protein degradation pathways are also being studied, many in combination with various chemotherapeutic regimens. The ability of docetaxel and, potentially, other agents to slow disease progression and improve survival in patients with AIPC indicates that the natural history of prostate cancer can be altered even in late stages of disease.
Unfortunately, these data also indicate that there is no clear standard of care for the large number of men who will develop a rising PSA while on ADT. Those patients who are symptomatic should undergo radiographic studies to assess for metastatic disease, and a full evaluation of known prognostic factors can give insight into a patient's future clinical course. Most importantly, patients should be evaluated for inclusion into a clinical trial, as there remains much to learn about the optimal treatment of patients with AIPC.
The algorithm in Figure 2 suggests an approach that can be taken when deciding how to manage patients with rising PSA despite ADT.
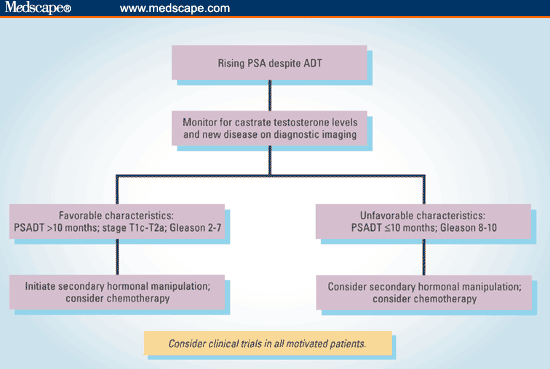
Figure 2. Suggested algorithm for approaching the patient with a rising PSA despite ADT.
Sunday, 19 February 2012
Handling relapse after Prostate Cancer Treatment - 2
Defining biochemical recurrence
Table 2: Guidelines for determining biochemical recurrence
| ||
Initial therapy
|
PSA threshold
|
Comments
|
Radical prostatectomy
|
0.2 ng/ml on at least two successive tests
|
Some physicians continue to use a higher threshold of 0.4 ng/ml or greater
|
Radiation therapy (external beam or brachytherapy)
|
Three successive elevations in PSA compared to nadir (low point), regardless of actual reading, according to the American Society for Therapeutic Radiology and Oncology
|
Many oncologists use a working definition that biochemical recurrence has occurred if PSA levels are greater than 1–2 ng/ml 12 to 18 months following initial treatment.
Ideally, post-treatment PSA levels should be less than 0.5 ng/ml, but this is rare; levels of 0.6–1.4 ng/ml may occur.
|
Neoadjuvant hormone therapy and radiation therapy
|
Unknown
| |
Further muddying the water, it is not clear what PSA levels should be in men who have undergone neoadjuvant hormone therapy in addition to radiation therapy. Hormone therapy suppresses levels of testosterone; once the therapy is stopped, testosterone levels rise, and PSA generally increases rapidly until the hormonal environment stabilizes.
A common challenge
Biochemical recurrence after surgery
Pound CR, Partin AW, Eisenberger MA, et al. Natural History of Progression after PSA Elevation Following Radical Prostatectomy. Journal of the American Medical Association 1999;281:1591–7. PMID: 10235151.
Roehl KA, Han M, Ramos CG, et al. Cancer Progression and Survival Rates Following Anatomical Radical Retropubic Prostatectomy in 3,478 Consecutive Patients: Long-Term Results. Journal of Urology 2004;172:910–14. PMID: 15310996.
|
Other studies indicate that a similar (or perhaps slightly higher) percentage of men treated with radiation therapy will experience a biochemical recurrence (see “Biochemical recurrence after radiation therapy,” below).
Biochemical recurrence after radiation therapy
Potters L, Morgenstern C, Calugara E, et al. 12-Year Outcomes Following Permanent Prostate Brachytherapy in Patients with Clinically Localized Prostate Cancer. Journal of Urology 2005;173:1562–6. PMID: 15821486.
Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of Conventional-Dose vs High-Dose Conformal Radiation Therapy in Clinically Localized Adenocarcinoma of the Prostate: A Randomized Controlled Trial. Journal of the American Medical Association 2005;294:1233–9. PMID: 16160131.
|
Sunday, 12 February 2012
How to handle a relapse after treatment for prostate cancer - Part One
“Am I going to die?”
For many men, it’s as if they’re dealing with another diagnosis of cancer, except this time it’s much worse because there is less likelihood of getting cured.
Table 1: Predictors of biochemical recurrence at time of diagnosisAlthough a number of clinical factors contribute to your risk of relapse after treatment, the parameters below provide a simpler assessment of your chances of biochemical recurrence, based on your clinical profile at the time of diagnosis.For more sophisticated estimates, based on specific risk factors, see Figures 1 through 3 ____________________________________________________________________________________________________________ | |
| Low risk (33% chance of biochemical recurrence within five years) | Gleason score less than or equal to 6 and PSA less than or equal to 10 ng/ml and Cancer stage T1c or T2a. |
(50% chance of biochemical recurrence
within five years)
Gleason score of 7 (if 3+4)
and/or PSA greater than 10 but no greater
than 20 ng/ml
and/or Cancer stage T2b.
</></></> High risk
(85% chance of biochemical recurrence within
five years)
Gleason score of 7 (if 4+3), or 8 or more
and/or PSA greater than 20 ng/ml
and/or Cancer stage T2c or more
</></></>________________________________________________________________________________________________
For those who have already suffered a biochemical recurrence after being treated for prostate cancer — or dread each follow-up blood test because it might signal such a recurrence — the next article in this series explains what a rising PSA after treatment really means and what your treatment options are.
Monday, 3 August 2009
Key Event In Prostate Cancer Progression Discovered
The onset of hormone-independent growth marks an advanced and currently incurable stage of prostate cancer.
The study, published in the July 24, 2009, issue of the journal Cell, focuses on androgen receptors, molecules located in the nucleus of cells of the prostate gland and other tissues. Male sex hormones – androgens – bind with these receptors to activate genes that control cell growth.
The researchers show that in androgen-independent prostate cancer, androgen receptors are reprogrammed to regulate a group of genes involved in a different, later, phase of cell division, triggering rapid cell growth. They further show that a modification of a chief component of the chromosome is responsible for this reprogramming.
"Some late-phase prostate cancer does not require androgen hormones for tumor growth, but it does require androgen receptors," says first author and co-corresponding author Qianben Wang, assistant professor of molecular and cellular biochemistry and a researcher with the Ohio State University Comprehensive Cancer Center - James Cancer Hospital and Solove Research Institute.
"Our study reveals the role of androgen receptors in hormone independent prostate cancer, how they become active in that disease and what genes they regulate to promote tumor growth."
The findings provide a better understanding of prostate cancer and could identify new therapeutic targets and lead to new treatments for this lethal stage of the disease, he says.
Prostate cancer is the most frequently diagnosed cancer in men. An estimated 192,280 new cases are expected in the United States in 2009, along with 27,360 deaths from the disease.
To conduct the study, Wang working with corresponding author Dr. Myles Brown, professor of medicine at Harvard Medical School and Dana-Farber Cancer Institute, and a group of colleagues used hormone-dependent and hormone independent prostate cancer cell lines, gene expression data and tissue from human tumors.
They showed that in hormone-dependent disease, androgen receptors regulate an early phase of cell cycle. In hormone-independent prostate cancer, however, the receptors are reprogrammed to selectively regulate genes involved in actual cell division, that is, the mitotic phase of the cycle.
A gene called UBE2C was a standout among these genes, and increased expression of that gene correlated with progression to the hormone-independent phase.
Furthermore, a chemical change – an epigenetic change – in a histone protein associated with that gene enabled androgen receptors to bind with and activate the gene in hormone-independent prostate cancer.
Finally, they show that over-expression of this gene is necessary for the growth of the hormone-independent prostate cancer cells.
"Interestingly," Wang says, "the UBE2C gene is also over-expressed in breast, lung, ovary, bladder, thyroid and esophageal cancers, suggesting that our findings could have wide application."
Funding from the National Cancer Institute and Department of Defense supported this research.
Adapted from materials provided by Ohio State University Medical Center, via EurekAlert!, a service of AAAS.
Monday, 22 June 2009
Longer Hormone Treatment May Improve Prostate Cancer Outlook
WEDNESDAY, June 10 (HealthDay News) -- Men with moderately advanced prostate cancer who get hormone-blocking drugs after radiation therapy do better when the drug treatment is continued for two or more years after an initial six-month regimen, a European study has found.
The results pretty much mirror those of a similar American trial reported in May, said Dr. Eric M. Horwitz, acting chairman of radiation oncology at Fox Chase Cancer Center in Philadelphia, who led the group that did the U.S. study.
"We have long believed that longer-term hormone therapy is the standard of care," Horwitz said. "These studies support that belief."
The results apply to men whose cancer shows signs of growth but has not spread beyond the prostate gland -- perhaps a quarter of all cases of prostate cancer, Horwitz said.
Earlier studies in the United States and Europe established the value of radiation therapy followed by six months of hormone-blocking treatment in such cases, he said. The new studies were designed to determine whether continuation of drug therapy that blocks the cancer-promoting activity of the male hormone testosterone could improve those results.
Though the studies differed in size and length, their results were similar in most respects.
The European trial, reported in the New England Journal of Medicine, included 970 men who were assigned to radiation therapy followed by either six months or three years of hormone-suppressing treatment. The five-year death rate of men in the longer-treatment group was 15.2 percent, compared with 19 percent for those in the shorter-term treatment group.
The U.S. study, published in the Journal of Clinical Oncology, included 1,554 men who were followed for 10 years. The study found no significant difference in overall survival -- 51.6 percent for the short-term group, given four months of treatment, and 53.9 percent for the long-term group, treated for two years.
But it did find a difference among men who were alive and cancer-free after 10 years. The disease-free survival rate for the short-term group was 13.2 percent, compared with 22.5 percent for those treated longer.
Other measures, such as the spread of cancer to other parts of the body and greater growth of the malignancy within the prostate gland, were consistently better for men in the U.S. study who'd had the longer-term therapy.
Horwitz said that differences between the American and European results were not unexpected. Similar differences had been found in the studies that established the value of the radiation-plus-hormone therapy, he said. One possible explanation, he said, is that prostate cancers tend to be diagnosed at an earlier stage in the United States because of extensive screening programs.
Both studies reported the expected side effects of hormone-blocking therapy, including hot flashes, weight gain, osteoporosis and loss of sexual function.
"Some men get them and some do not," Horwitz said. "Over the last few years, there has been a lot of attention paid to these side effects, in the medical literature and in public awareness, and there has been more reluctance to use this therapy. These studies clearly identify a group of men who benefit from this therapy."
But Dr. Peter C. Albertsen, chairman of urology at the University of Connecticut Health Center, said that it's those side effects that should limit the use of longer hormone-blocking therapy to a specific group of men with prostate cancer -- those with "disease that is clinically evident on palpation [by touching] but with no evidence that it has spread outside the prostate," Albertsen wrote in an accompanying editorial in the journal.
He agreed that earlier forms of the cancer are more often detected in the United States than in Europe because of extensive screening programs. The side effects that balance the benefits of hormone-blocking therapy rule against extended therapy for those men, Albertsen said.
"For men with localized disease that is screening-detected, the equation is quite different," he said.
More information:
The U.S. National Cancer Institute has more on prostate cancer.
New Approach For Treating Recurrent Prostate Cancer On The Horizon
ScienceDaily (June 15, 2009) — A new study shows that an alpha-particle emitting radiopeptide—radioactive material bound to a synthetic peptide, a component of protein—is effective for treating prostate cancer in mice, according to researchers at SNM's 56th Annual Meeting in Toronto. The results could eventually result in a significant breakthrough in prostate cancer treatment, especially for patients whose cancer recurs after the prostate is removed.
"Our study shows that this novel form of treatment has the potential to target and destroy cancer cells with minimal damage to surrounding healthy tissue," said Damian Wild, University Hospital Basel, Basel, Switzerland, lead author of the study. "Eventually, this therapy could give hope to some of the hardest-to-treat prostate cancer patients and also could be applied to other types of cancer."
Every year, more than 186,000 men in the United States are newly diagnosed with prostate cancer. The most common types of treatment include surgical removal of some, or all, of the prostate, followed by radiation therapy. More than 30,000 men each year who have had their prostates removed experience recurrence of the cancer. In most of these cases, the disease cannot be localized and treated adequately with conventional treatments; therefore, a systemic treatment that efficiently kills small tumors is needed.
Because tumor cells readily bind with certain peptides, researchers have been able to develop highly specific radiopeptides that bind with tumor cells and treat them using specific therapeutic radioactive substances attached to the radiopeptide. Prostate cancer cells—and many other types of cancer cells—have an overabundance of gastrin-releasing peptide receptors, making the cancer a strong candidate for treatment with radiopeptides.
The study compared two different types of radiopeptides. One group of mice was injected with 213 Bi-DOTA-PESIN, which emits alpha particles that are effective at killing cancer cells. The other group was injected with beta-emitting 177 Lu-DOTA-PESIN, which are also effective in tumor cell-killing, but can also cause damage to nearby healthy cells. Alpha particles are able to kill cancer cells without damaging surrounding healthy tissue. A third group of mice received no treatment.
However, at the maximum tolerated dose, the alpha-emitting 213 Bi-DOTA PESIN was significantly more effective, tripling the survival rate of the mice that received the therapy. The results indicate that the alpha-emitting radiopeptide could provide a new approach for treating prostate cancer and eventually other types of cancer.
Adapted from materials provided by Society of Nuclear Medicine.
Saturday, 20 June 2009
Dramatic Outcomes In Prostate Cancer Study
ScienceDaily (June 19, 2009) — Two Mayo Clinic patients whose prostate cancer had been considered inoperable are now cancer free thanks in part to an experimental drug therapy that was used in combination with standardized hormone treatment and radiation therapy. The men were participating in a clinical trial of an immunotherapeutic agent called MDX-010 or ipilimumab.
In these two cases, physicians say the approach initiated the death of a majority of cancer cells and caused the tumors to shrink dramatically, allowing surgery. In both cases, the aggressive tumors had grown well beyond the prostate into the abdominal areas.
"The goal of the study was to see if we could modestly improve upon current treatments for advanced prostate cancer," says Eugene Kwon, M.D., Mayo Clinic urologist and leader of the clinical trial. "The candidates for this study were people who didn't have a lot of other options. However, we were startled to see responses that far exceeded any of our expectations."
The patients first received a type of hormone therapy called androgen ablation, which removes testosterone and usually causes some initial reduction in tumor size. Researchers then introduced a single dose of ipilimumab, an antibody, which builds on the anti-tumor action of the hormone and causes a much larger immune response, resulting in massive death of the tumor cells.
Both men experienced consistent drops in their prostate specific antigen (PSA) counts over the following weeks until both were deemed eligible for surgery. Then, during surgery, came a greater surprise.
"The tumors had shrunk dramatically," says Michael Blute, M.D., Mayo urologist, co-investigator and surgeon, who operated on both men. "I had never seen anything like this before. I had a hard time finding the cancer. At one point the pathologist (who was working during surgery) asked if we were sending him samples from the same patient."
One patient underwent radiation therapy after surgery; both have resumed their regular lives. Further research is being planned to understand more about the mechanisms of the antibody and how best to use the approach in practice. The researchers, however, note the significance of their findings.
"This is one of the holy grails of prostate cancer research," says Dr. Kwon. "We've been looking for this for years."
The research was supported by the Department of Defense, The Richard M. Schulze Family Foundation, the Mayo Clinic Cancer Center and the Mayo Clinic Center for Translational Science Activities. Medarex, Inc. provided the study drug free of charge and supported safety monitoring during the protocol.
Source: Mayo Clinic (2009, June 19). Dramatic Outcomes In Prostate Cancer Study.
Thursday, 11 June 2009
Hormone Therapy May Confer More Aggressive Properties To Prostate Tumors
This is one of the conclusion of a thesis presented at the Sahlgrenska Academy, University of Gothenburg, Sweden.
Hormone therapy has serious side effects and is therefore used only when the tumour has grown too large to be treated in any other way, or when the tumour has spread and formed metastases. The hormone that is given causes the natural production of male sex hormone to fall, and the tumour stops growing. Pain also usually decreases.
"Our results suggest that the tumour properties change following hormone therapy such that the tumours at a later stage can continue to grow and spread in the body. For this reason, it is probably necessary to supplement the hormone therapy in order to compensate for these changes", says pharmacist Karin Jennbacken, author of the thesis.
The results show that patients who have been given hormone therapy have higher levels of the proteins that enable the cancer cells to move through the body and attach to other organs. One of these proteins is known as "N-cadherin", and this protein is present in higher levels in patients who have been given hormone therapy.
"We don't have any good treatment alternatives in cases where the tumour returns after hormone therapy, and this means that it is particularly important to study how such tumours are controlled and how they behave. The properties that we have identified may become targets for new anti-metastatic drugs in advanced prostate cancer", says Karin Jennbacken.
Prostate Cancer
Approximately 9,000 new cases of prostate cancer are diagnosed each year in Sweden, making it the most common of all cancer forms. Many of the tumours grow very slowly and give no symptoms, but prostate cancer can also display a more aggressive course, spreading metastases to lymph nodes, the skeleton and other locations. The complete prostate is often surgically removed if the cancer is diagnosed early. Other treatments available are radiation therapy and hormone therapy.
Title of the thesis: Invasive and metastatic properties of advanced prostate cancer.
Adapted from materials provided by University of Gothenburg.
Wednesday, 3 June 2009
From Prostate Imaging to Prostate Cancer Imaging
John P. Michael Sedelaar, PhD, MD, a postdoctoral research fellow at Johns Hopkins University, presented these findings at the American Association for Cancer Research 100th Annual Meeting 2009, noting that “By adding a radioactive imaging probe in these compounds, we can combine therapeutics with diagnostic imaging.”
The study, conducted in mice, employed the drug thapsigargin, a nonspecific, highly cytotoxic agent. The researchers added a tyrosine ring to this agent for the coupling of imaging probes. Once in the body, this prodrug is made active by proteins—either prostate-specific membrane antigen (PSMA) or prostate-specific antigen (PSA)—which are more prominently present in prostate cancer, and even more prevalent in highgrade prostate cancer and metastasis.
“This inactivated compound has an amino acid tail specifically chosen so that it can only be ‘clipped' by PSA or PSMA,” Dr Sedelaar explained in an interview with Oncology Nursing News. “When this tail is clipped off, the chemical compound is released and activated and can be taken up into the cell and be therapeutically active.”
“It's like a smart bomb, to use a military analogy,” he continued. “By retooling chemotherapy agents, we may be able to get more accurate treatment monitoring and follow-up.”
Unlike other targeted therapies, this treatment is based upon the general principles of prostate cancer, not the individual patient's genetic makeup. “These smart bombs we're developing are not `tailor-made,'” noted Dr Sedelaar; rather, they are based on “the fact that prostate cancers have elevated amounts of PSA and PSMA.”
The need for targeted approaches to prostate cancer is essential, according to Dr Sedelaar. “An increasing number of patients have minimal prostate cancer, and opt for either very focused treatment or the watchful waiting approach,” he noted. “In this environment, the need for an accurate imaging tool is paramount.”
In terms of the study results, singlephoton emission computed tomography imaging of the tumor-bearing mice showed uptake by tumors together with uptake by thyroid, liver, and spleen. The PSMA imaging drug was also detectable in the kidneys and bladder. No toxicity was noted; and even more importantly, there was a measurable reduction in prostate cancer cells.
When asked whether this approach might be transferable to other cancers and other drugs, Dr Sedelaar would say only that “there could be possibilities to retool the compounds for other chemotherapeutic agents, but we haven't reached that stage yet.”
Dr Sedelaar told Oncology Nursing News that a small multicenter trial of these PSMA therapies will start this year, but in terms of the imaging compounds, “We're still in the animal experiments. The compounds are not yet as specific as we want them to be.” He could provide no details about future human trials.
ASCO: Gene Test Predicts Prostate Cancer
In a case-control study, the six-gene panel outperformed a standard test -- age-adjusted prostate specific antigen -- in distinguishing between men with cancer and those without, according to Robert Ross, M.D., of Dana-Farber Cancer Institute in Boston.
When prostate specific antigen (PSA) levels were added to the group of genes, the performance of the test improved even more, Dr. Ross told attendees at the annual meeting of the American Society of Clinical Oncology.
The gold standard for diagnosing prostate cancer is a biopsy, he said, but 60% of biopsies in men thought to be at risk for the disease turn out to be negative.
The goal of this test is to avoid the "pain, discomfort, and anxiety" associated with biopsies, Dr. Ross said, by winnowing out the 60% of men who don't need the procedure.
Dr. Ross and his colleagues started with a set of 392 genes associated with inflammation, cancer, and the epidermal growth factor receptor, as well as some identified in other genetic studies of cancer.
In a training set of 76 healthy men and 76 with prostate cancer, six genes were significantly associated with disease. Of the six, five are less active in those with the disease and one has greater activity, the researchers found.
The finding was validated in a second cohort of 128 men with cancer and 94 without, he said.
In the second group, the six-gene test correctly detected 85.9% of the men with disease, compared with 69.5% detected by age-adjusted PSA, Dr. Ross said.
The six-gene test had a specificity of 83%, compared with 93.6% for PSA, he said.
When the researchers did both the six-gene test and measured PSA levels, the sensitivity and specificity improved -- to 87.5% and 92.6%, respectively.
The results are a "significant improvement" over the predictive value of PSA alone, Dr. Ross said.
"From a clinical hypothesis standpoint, this is great data," Dr. Ross said. But, he cautioned, "this is a case-control study (and) you'd like to see it validated prospectively."
He said his institution and several others are collaborating on a 1,000-patient prospective study -- dubbed PRECISE -- among men who meet the criteria for a biopsy, but have not yet had the test.
The goal will be to see if the test can predict the results of biopsy, he said.
Although the test is still in development, it will not be expensive, especially compared with the $2,000 it costs for a biopsy, said Karl Wassman, of Source MDx, the Boulder, Colo. company that has developed the test.
He said the blood test can be read by standard equipment, using a kit of primers and probes developed by Source MDx, so that the cost will be in the range of "a couple of hundred" dollars.
The various forms of PSA testing are "extremely valuable" in screening for prostate cancer, said Howard Sandler, M.D., of Cedars-Sinai Medical Center in Los Angeles, who was not involved in the study.
But the jury is out on whether screening and early detection have any benefit for patients, he said.
"If screening is beneficial, then better screening is important," he said, but there's no high-quality evidence that early detection is useful.
Dr. Sandler said the "weakness of this test" is that it doesn't answer the most important question about prostate cancer.
"The question is do you have clinically relevant prostate cancer or not?" he said. "Do you have potentially lethal cancer or do you have the cancer that will never kill you?"
Dr. Ross agreed that that question is important and said he and his colleagues have preliminary data that suggests it may be possible to use such a test to distinguish between types of cancer.
The study was supported by Source MDx, Dana Farber and the Harvard Cancer Center, the Gelb Center, and the Bing Sound Wong Fund.
Several researchers reported financial links with Source MDx.
Dr. Sandler reported financial links with sanofi-aventis, Genentech, Amgen, and AstraZeneca.
Primary source: Journal of Clinical Oncology
Source reference:
Ross RW, et al "Sensitivity and specificity of a whole-blood RNA transcript-based diagnostic test for the diagnosis of prostate cancer (CaP) compared with prostate-specific antigen (PSA) alone" J Clin Oncol 2009; 27(15S): Abstract 5052.
Friday, 29 May 2009
New Blood Test Greatly Reduces False-positives In Prostate Cancer Screening
ScienceDaily (May 28, 2009) — A new blood test used in combination with a conventional prostate-specific antigen (PSA) screening sharply increases the accuracy of prostate cancer diagnosis, and could eliminate tens of thousands of unneeded, painful, and costly prostate biopsies annually, according to a study led by researchers at Dana-Farber Cancer Institute.
At the annual meeting of the American Society of Clinical Oncology in Orlando, Fla., William K. Oh, M.D., and Robert W. Ross, M.D., will report that the six-gene molecular diagnostic test, when combined with a PSA test, accurately detected prostate cancer more than 90 percent of the time. Earlier studies suggest that the conventional PSA test is 60-70 percent accurate in detecting cancer. The findings will be discussed at a poster session on May 31.
Men who are found to have elevated levels of PSA in routine screening tests are often referred for a biopsy of the gland to check for tumors. Nearly two-thirds of biopsies performed -- a painful procedure with some risk of complications -- do not find any cancerous cells. This high rate of "false positive" PSA test results underscores the need for a more accurate method for detecting prostate cancer, said Oh, who is the clinical director of the Lank Center for Genitourinary Oncology at Dana-Farber.
The two-year study involved 484 participants. The group comprised 204 men with known prostate cancer, 110 men with benign prostatic hypertrophy (BPH), and 170 healthy men in a control group. (BPH can elevate PSA levels in the blood, which often leads to a biopsy to rule out prostate cancer.) These groups were split into age-matched training and validation sets.
The researchers sought to measure the accuracy of a six-gene whole blood RNA transcript-based diagnostic test developed by Source MDx in Boulder, Colo., both in terms of its sensitivity (the ability to detect prostate cancer) and specificity (the ability to identify people who don't have prostate cancer).
Source MDx researchers developed the test after initially working with a set of 174 candidate genes whose activity was compared in the different study groups. They narrowed the pool down to just six genes that, as a group, were highly sensitive in predicting which patients had prostate cancer and which were normal.
The study found that "the six-gene model was more accurate than PSA alone at predicting cancer if you had it and no cancer if you didn't," said Oh. The test's accuracy improved even more when PSA measurements were added. Combined, the two tests achieved a diagnostic accuracy of more than 90 percent in specificity and sensitivity and eliminated most of the false-positives yielded by the PSA test.
Based on these findings, the researchers are planning to conduct a larger, multicenter clinical trial involving approximately 1,000 men to determine if the findings remain valid.
"These findings are very encouraging and suggest that this new test could spare tens of thousands of men from undergoing an unnecessary biopsy," Oh said. "However, until we can verify our findings, it is important to recognize that the PSA test, despite its limitations, is still the best test available for diagnosing prostate cancer at this time."
The study was funded in part by Source MDx and a Prostate Cancer SPORE grant at Dana-Farber/Harvard Cancer Center.
Wednesday, 27 May 2009
Carbohydrate Restriction May Slow Prostate Tumor Growth
ScienceDaily (May 26, 2009) — Restricting carbohydrates, regardless of weight loss, appears to slow the growth of prostate tumors, according to an animal study being published this week by researchers in the Duke Prostate Center.
"Previous work here and elsewhere has shown that a diet light in carbohydrates could slow tumor growth, but the animals in those studies also lost weight, and because we know that weight loss can restrict the amount of energy feeding tumors, we weren't able to tell just how big an impact the pure carbohydrate restriction was having, until now," said Stephen Freedland, M.D., a urologist in the Duke Prostate Center and lead investigator on this study.
The researchers believe that insulin and insulin-like growth factor contribute to the growth and proliferation of prostate cancer, and that a diet devoid of carbohydrates lowers serum insulin levels in the bodies of the mice, thereby slowing tumor growth, Freedland said.
Animals in the study were fed one of three diets: a very high fat/ no carbohydrate diet; a low-fat/ high carbohydrate diet; and a high fat/ moderate-carbohydrate diet, which is most similar to the "Western" diet most Americans eat, Freedland said. They were then injected with prostate tumors at the same time.
"The mice that were fed a no-carbohydrate diet experienced a 40 to 50 percent prolonged survival over the other mice," Freedland said.
Mice on the no-carbohydrate diet consumed more calories in order to keep body weights consistent with mice on the other study arms.
"We found that carbohydrate restriction without energy restriction – or weight loss – does indeed result in tumor growth delay," he said.
The researchers plan to begin recruiting patients at two sites – Duke and the University of California – Los Angeles – for a clinical trial to determine if restricting carbohydrate intake in patients with prostate cancer can similarly slow tumor growth. The trial should begin within a few weeks.
"It's very exciting – this is a potential new mechanism to fight prostate cancer growth and help patients live longer with their disease," Freedland said.
The findings appear in the May 26, 2009 online edition of the journal Cancer Prevention Research. Funding was provided by the United States Department of Veterans Affairs, the Department of Defense Prostate Cancer Research Program; the American Urological Association/ Foundation Astellas Rising Star in Urology Award, and the Robert C. Atkins Foundation. The Atkins Foundation supported this study but had no additional input or influence on the results.
Other researchers involved in this study include John Mavropoulos, William Buschmeyer, Alok Tewari, Dmitriy Rohkfeld, Phillip Febbo, Gayathri Devi, Eric Westman, Bercedis Peterson and Salvatore Pizzo of Duke; Michael Pollak and Yunhua Zhao of McGill University; Pinchas Cohen and David Hwang of UCLA and Wendy Demark-Wahnefried of the University of Texas – M.D. Anderson Cancer Center.
Saturday, 23 May 2009
Protein That Suppresses Androgen Receptors Could Improve Prostate Cancer Diagnosis, Treatment
ScienceDaily (May 20, 2009) — A protein that helps regulate expression of androgen receptors could prove a new focal point for staging and treating testosterone-fueled prostate cancer, Medical College of Georgia researchers say.
Levels of the protein, βarrestin2, are lower in some prostate cancer cells than in normal prostate cells while expression of testosterone-fed androgen receptors is higher, they recently reported in Proceedings of the National Academy of Sciences Online Early Edition.
"An increase in the number of androgen receptors is believed responsible for prostate cancer progression in men with advanced disease," says the study's corresponding author, Dr. Yehia Daaka, Distinguished Chair in Oncologic Pathology in the MCG School of Medicine.
With increased numbers of androgen receptors, prostate cancer can make use of the limited testosterone available after a diseased prostate gland is removed or after testosterone production is blocked by drug therapy. In fact, the increased number of androgen receptors may mutate so they can start feeding off other steroids or even growth factors, Dr. Daaka says.
These wily skills help explain why cancer returns despite initially promising treatment results.
"It is clear that signaling by the androgen receptor is paramount for not only the initiation but also the progression of the disease, including escape to a hormone-refractory disease," he says. Moves androgen receptors make to support cancer growth make it "unbeatable at this point," for some patients.
However increased levels of βarrestin2 appear to halt the potentially deadly increase in androgen receptor expression, the MCG research team has found.
Androgen receptors have co-factors that can activate or repress their activity. "You could make the leap and say perhaps prostate cancer initiation and progression may be regulated by expression or non-expression of these co-factors," says Dr. Daaka, a Georgia Cancer Coalition Distinguished Cancer Scholar.
Their studies in human tissue – both in culture and transplanted into mice – show this appears the case for βarrestin2. First the team identified βarrestin2 as cofactor for androgen receptors. Next they found a reciprocal relationship: androgen receptor expression is low when βarrestin2 expression increases. That's the scenario in healthy prostate cells while the exact opposite is true in some prostate cancer. When they forced increased expression of βarrestin2, androgen receptor expression and activity went down.
βarrestin2 locks up an androgen receptor by binding to it, then the pair bind to yet another protein, ubiquitin ligase, which tags the receptor as waste and the trio make their way to the cell's garbage dump. "The neat thing about it is βarrestin2 inhibits or blunts the androgen receptor by promoting its degradation. So it disappears," Dr. Daaka says.
His future studies include determining what happens when βarrestin2 expression is further decreased in the face of prostate cancer. These studies will also help determine how big a player βarrestin2 is in prostate cancer progression, says Dr. Daaka, noting that numerous other corepressors and activators of androgen receptors are known.
Since all the happenings occur inside prostate cells, the findings don't point toward a new blood or urine test for prostate cancer but could lead to new ways to stage prostate cancer from the first biopsy. In fact, Dr. Daaka and his team already are collecting samples from patients whose cancer has been staged to see if specific levels of βarrestin2 expression correlate with different stages of disease.
Another goal is to develop a small molecule that can get inside a patient's cell and mimic βarrestin2's ability to suppress androgen receptor expression and so restore healthy levels found in prostate cells.
Prostate cancer falls behind skin cancer as the second most common cancer in men and more than 192,000 new cases will be diagnosed this year in the United States, according to the American Cancer Society.
Collaborators include Dr. Vijayabaskar Lakshmikanthan, postdoctoral fellow; Dr. Lin Zou, former postdoctoral fellow; Jae Kim, graduate student; Dr. Nidia C. Messias, assistant professor; and Dr. Zhongzhen Nie, assistant professor; from the MCG Department of Pathology; and Drs. Allison Michal and Jeffrey L. Benovic from Thomas Jefferson University.