Unfortunately, the natural history of these patients is poorly understood. Numerous factors such as the lactate dehydrogenase, alkaline phosphatase, hemoglobin, and Eastern Cooperative Oncology Group or Karnofsky performance status have been used to predict prognosis,[39,40] while others have suggested that a detailed PSA history alone may be enough to stratify risk and predict prognosis.[41]
Because the velocity of PSA changes may be different before and after ADT, reflecting changes in tumor kinetics after treatment, intermediate end points based on the ratio of the post- to pre-ADT slope are being considered in clinical trials as an end point for comparing systemic therapy in patients with the "lethal phenotype" (ie, PSA doubling time < 3 months).
Nevertheless, until PSA doubling time and/or calculated pre- and post-ADT PSA velocities as predictors of survival in patients are widely accepted, a 50% decline in PSA is typically considered the benchmark for response to ADT, while PSA increases of a minimum of 5 ng/mL with two consecutive increases of 25% after ADT indicate clinical disease progression.[2,38,42]
Treatment Strategies
Castrate patients with a rising PSA present unique and complex challenges for the treating physician. Frequently, ADT continues to be employed while additional therapeutic strategies are explored. At this stage of disease, the establishment of a true multidisciplinary care team is essential, including the coordinated involvement of a urologist, an oncologist, and a radiologic imaging specialist.Before any additional therapeutic regimens can be considered, maintenance of castrate levels of testosterone (< 50 ng/mL) should be confirmed, as the presence of a rising PSA may in fact indicate that ADT is not sufficiently suppressing testosterone production and/or action and that additional androgen suppression therapy is warranted.[2]
Even if androgen independence is established despite castrate levels of testosterone and the rising PSA levels indicate biochemical failure, the tumor might respond to secondary hormonal manipulation.
In patients who had been treated with an antiandrogen, withdrawal of the agent while maintaining castrate levels of testosterone has been associated with PSA responses, as well as with symptomatic and objective responses, in about 25% of patients. The duration of response is typically 3-4 months, but some cases have lasted for several years.[2] This response has been noted with flutamide, bicalutamide, and nilutamide[43] and may result from mutations of androgen receptors.[44]
Thus, a trial of antiandrogen withdrawal may be reasonable for certain patients, particularly before initiating more toxic therapy. Although it is unclear whether the same antiandrogen would still be effective if reintroduced at a later point, cross-reactivity generally does not exist among the antiandrogens: both bicalutamide and nilutamide have shown some activity as secondary antiandrogens following resistance to flutamide.[45-47]
Adrenal androgen inhibitors, most commonly ketoconazole, also have a role as second-line hormonal agents. Approximately 10% of circulating androgens derive from the adrenal glands, and a higher proportion of adrenal androgens may be in prostate cancer cells.[2] Ketoconazole has been shown to decrease serum PSA by 50% with high doses[48] and low doses,[49] but the duration of response is short, with one study finding a median PSA response duration of only 3.5 months.
Similarly, glucocorticoids have also shown some effect in patients with AIPC. In several trials comparing hydrocortisone alone with hydrocortisone and a study treatment, disease response was seen in the hydrocortisone-alone arms.[50-53] However, as with ketoconazole, the duration of response tends to be rather short and further progression of disease is rapidly seen.
A more promising approach has been seen with chemotherapeutic agents. Chemotherapy with cytotoxic agents historically has had a minor role in the treatment of prostate cancer. In 1985, Eisenberger and colleagues reviewed 17 randomized clinical trials and found complete and partial response rates in only 4.5% of patients.[54] In 1993, in another large review, the overall response rate with chemotherapy was 8.7%.[55]
Switching tactics, researchers began to measure quality-of-life parameters in this population, and the use of mitoxantrone showed significant palliative benefits,[51] which led to its approval by FDA for use in patients with AIPC. Unfortunately, aside from its palliative benefits, it has shown only modest activity in measurable disease and lacks the ability to confer a significant survival advantage in patients with AIPC.[52]
By contrast, two phase 3 trials with docetaxel, one pairing with prednisone and the other pairing with estramustine, showed significant improvement in both survival and palliation in patients with AIPC when compared with the standard mitoxantrone/prednisone regimen.[56,57] These trials led to the recent approval by FDA of docetaxel in combination with prednisone every 3 weeks in patients with AIPC.
Newer agents targeting apoptosis, growth factors, angiogenesis, tumor-associated antigens, and protein degradation pathways are also being studied, many in combination with various chemotherapeutic regimens. The ability of docetaxel and, potentially, other agents to slow disease progression and improve survival in patients with AIPC indicates that the natural history of prostate cancer can be altered even in late stages of disease.
Unfortunately, these data also indicate that there is no clear standard of care for the large number of men who will develop a rising PSA while on ADT. Those patients who are symptomatic should undergo radiographic studies to assess for metastatic disease, and a full evaluation of known prognostic factors can give insight into a patient's future clinical course. Most importantly, patients should be evaluated for inclusion into a clinical trial, as there remains much to learn about the optimal treatment of patients with AIPC.
The algorithm in Figure 2 suggests an approach that can be taken when deciding how to manage patients with rising PSA despite ADT.
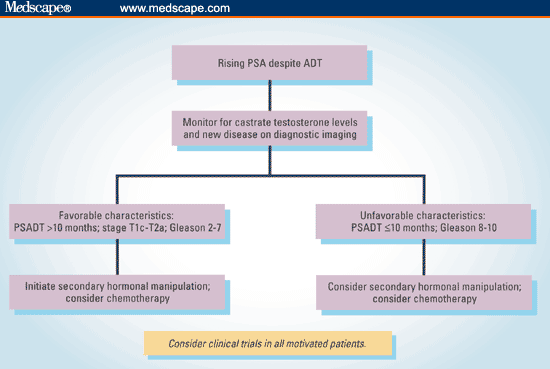
Figure 2. Suggested algorithm for approaching the patient with a rising PSA despite ADT.
2 comments:
Hi John! I just got reading through a few of your posts and I had a quick question. I am also a member of the cancer community and I was hoping you could email me back when you get the chance. Thanks! - emilywalsh688@gmail(dot)com.
Emmy
Hi Emily,
I will email you soon - John
Post a Comment